
Why shall we flush the combi boilers periodically?
What is limescale?
First of all,technically, the scale is mainly composed of calcium carbonate and magnesium carbonate salts, sounds a lot like limestone, right? Completely correct! Scale is basically limestone deposited in the water.
Absolutely pure water, chemical formula as H2O, is a colorless and odorless liquid at room temperature. However, there is no pure water in nature. Water will dissolve all substances in direct contact with it more or less. The characteristics of this "universal solvent" make natural water and municipal water supply and other residential water sources rich in various impurities. Impurities can cause a range of problems for water-based systems.
The types of impurities in water can be roughly divided into three categories:
1. dissolved solids
2. suspended solids
3. dissolved gas
How does scale form?
The hardness of water is usually measured in ppm (parts per million), which refers to the amount of calcium and magnesium salts dissolved in the water.It can be roughly converted to 1mg/L. For example, if the water hardness is 300ppm in some area, it can be said that the total amount of calcium carbonate and magnesium carbonate contained in 1 liter of water in this area is 300mg. "Hard water" usually refers to water containing more than 250 mg of calcium and magnesium salts per liter of water (as shown in the picture above, the WHO definition of hard water is more stringent), which is already high. Water hardness in many parts of the world is even much higher than this. The difference in water quality varies greatly according to the geological conditions and precipitation characteristics of each region.
The hardness salts naturally present in water are mainly calcium bicarbonate and magnesium bicarbonate. They are very soluble in water - which means it is normal for them to dissolve in water. However, if other factors change, such as temperature and pH, these hardness salts will undergo a series of changes. First, let's look at the temperature... As the temperature increases, calcium bicarbonate reacts:
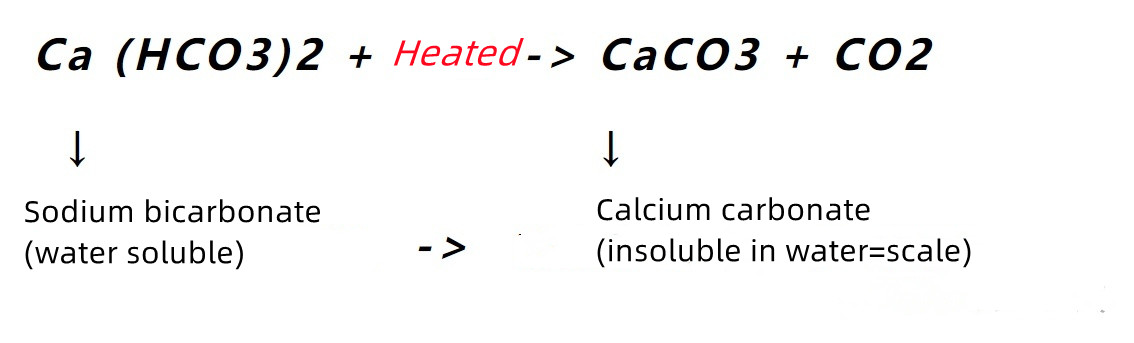
The biggest change in this process is that calcium carbonate [CaCO3] is much less soluble in water compared to calcium bicarbonate [Ca(HCO3)2]. Also, as the temperature increases, the solubility of calcium carbonate becomes even lower! The result: when high hardness water is heated, the result is the formation of solid calcium carbonate.
A combination boiler home heating system without protection, is actually a perfect scale production plant. Of course, since the combi boiler heat exchanger is the hottest area (where the heat is conducted from the flame to the water system), you will find that the heat exchanger is the most likely component to develop and be affected by scale. In addition to high temperatures, the pH of the water in the heating system can also have a large impact on the formation of scale.
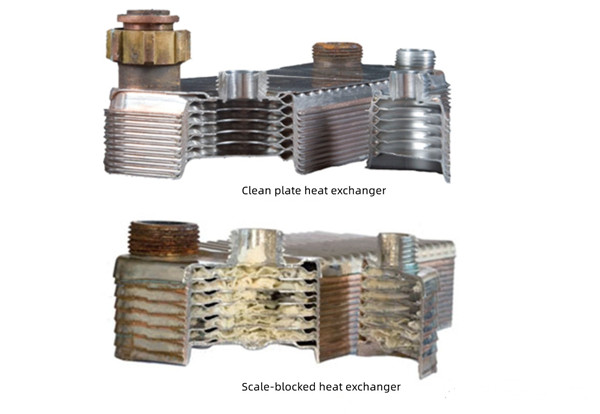
Problems caused by limescale
What problems can limescale inside a heating system cause? When scale forms and accumulates, it has two main harmful effects on the heating system:
Heating efficiency drops sharply
Formed in the hottest areas - especially the secondary heat exchangers of wall-hung boilers - plate heat exchangers.
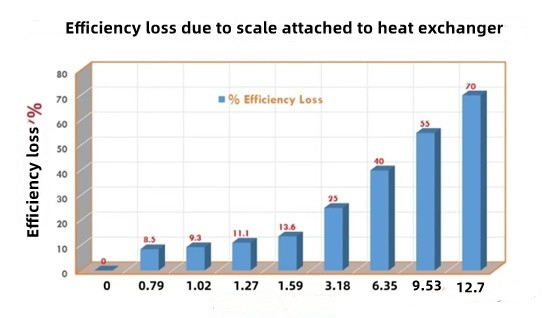
Very unfortunately limescale is a very poor conductor of heat! The thermal efficiency loss of the system caused by the adhesion of scales of different sizes on the heat exchanger.
Therefore, in order to ensure the comfort of heating, the boiler needs to consume gas for a longer time in order to continuously input the heat into the circulating system water, and finally achieve the purpose of raising the room temperature. This leads to the second problem -
Heating bills soar
Just a thin layer of scale - 1mm - can lead to an increase in gas usage (on gas bills) of more than 10%.
Eventually, vital components of the system can become so clogged with limescale that the heating system stops working completely. This means expensive repairs and may even require replacement of boiler parts.
How can we avoid limescale problems?
The good news is that there are now very simple and effective solutions to avoid scale formation in your system. Just make sure your engineer adds a high-quality chemical protectant to the heating system at the time of installation. And regularly check the protective agent content. Adding a high-quality protectant is much cheaper than the costly repairs and replacements that come with letting scale build up!
What should we do if scale problems have occurred in the system?
The good news is that this can be easily fixed by using an efficient system washer and a suitable, effective chemical cleaner.
Categories
New Blog
© Copyright: 2026 Globalsmartheater. All Rights Reserved.

IPv6 network supported